Medical Device
Today’s medical device companies are faced with constantly changing regulations like the FDA's UDI and the EU’s MDR systems, along with controlling costs and scaling operations. Labeling and Artwork Management play a key role in meeting these challenges.
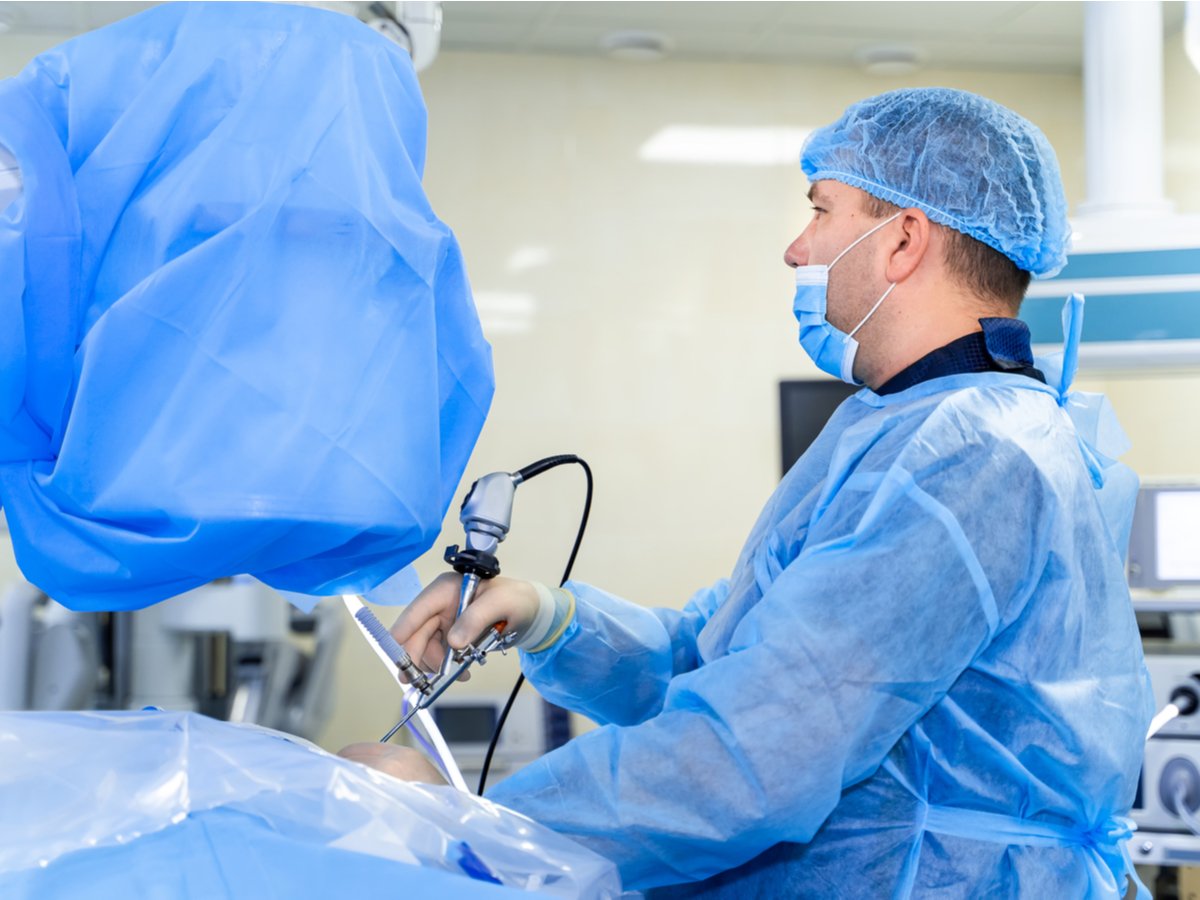





























Achieving sustainability in the life sciences supply chain
Want to find out how your life sciences organization can become more sustainable without compromising on quality or cost?
Download white paper todayKeep your labeling system in good health.
Enable regulatory compliance
Simplified Compliance. You need a validation-ready solution with built-in role-based access, document versioning, configurable approval workflows and electronic records and electronic signatures (ERES); all designed to ease compliance efforts.
Integrate sources of truth
Secure UDI DI and PI data. Avoid unnecessary risk when your source data from approved content management systems, trusted ERPs, and other applications that generate serial numbers and other data needed to meet regulatory requirements.
Centralize your data
Complete visibility. Your business may have many locations, but that doesn’t mean it should be “every entity for itself.” Centralized labeling brings visibility, control, and the ability to demonstrate compliance and meet corporate standards.
Enable label changes
Unburden IT. Business users should be able to create and manage medical barcode labels and configure business rules without calling on IT. This allows them to respond to any pressing regulations quickly.
Ensure security/auditability
Data that’s safe and sound. With business intelligence, full auditing and reporting capabilities allows you to monitor and track all labeling activity and eSignature capabilities so you can safely meet regulations.
Rethink legacy systems
Automate for Ease. Spreadsheets and other manual processes are not secure. Automated, validated labeling solutions can easily accommodate new requirements, safeguard against future regulations, and scale quickly.
Related resources
E-book
6 critical questions and answers on medical device labeling
This Q&A addresses key challenges that medical device companies face and how leveraging innovation in cloud labeling helps organizations succeed in a highly regulated, highly competitive global marketplace.
Blog
Why medical device manufacturers are embracing cloud-first labeling strategies
With cloud labeling, medical device companies improve regulatory compliance and avoid costly labeling errors, while maintaining a crucial level of safety customers and supply chains require. Additionally, organizations can be up and running quickly, benefit from automatic software updates, pay on a subscription basis, easily scale their printing operations when needed, reduce infrastructure costs, obtain advanced IT security, and improve their sustainability credentials.
Case Study
W.L. Gore enables compliance and accelerates go-to-market with cloud-based labeling
Learn how W.L. Gore has modernized and future-proofed labeling by migrating to Loftware Spectrum, and benefited from improved speed-to-market, automated label design and management to easily meet complex regulatory requirements and simplified auditing processes to enable compliance.